برقيو: جي ورجائن ۾ تفاوت
ڊاٿل مواد شامل ڪيل مواد
EmausBot (بحث | ڀاڱيداريون) م r2.7.2+) (Robot: Modifying vi:Electron |
Mathonius (بحث | ڀاڱيداريون) removing English texts |
||
| سِٽَ 6: | سِٽَ 6: | ||
!align="center" bgcolor=gray|برقيو |
!align="center" bgcolor=gray|برقيو |
||
|- |
|- |
||
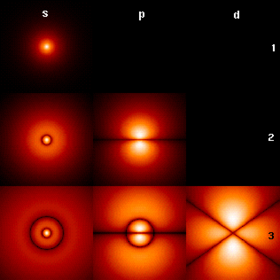
|align="center"|[[عڪس:HAtomOrbitals.png|center|thumb|280px|<big> <font face="MBSarang Sattar" size="4">پھريان چند برقياتي مدارچا [[ھائيڊروجن اڻو]] |
|align="center"|[[عڪس:HAtomOrbitals.png|center|thumb|280px|<big> <font face="MBSarang Sattar" size="4">پھريان چند برقياتي مدارچا [[ھائيڊروجن اڻو]]</big>]] |
||
|- |
|- |
||
!align="center" bgcolor=gray|<font face="MBSarang Sattar" size="4">درجھ بندي |
!align="center" bgcolor=gray|<font face="MBSarang Sattar" size="4">درجھ بندي |
||
| سِٽَ 40: | سِٽَ 40: | ||
|- |
|- |
||
| |
| |
||
| 0.510 998 918(44) [[MeV]]/[[روشنيءَ جي رفتار|c]]<sup>2</sup> |
| 0.510 998 918(44) [[MeV]]/[[روشنيءَ جي رفتار|c]]<sup>2</sup> |
||
|- |
|- |
||
|<font face="MBSarang Sattar" size="4">[[بنيادي چارج|برقي چارج]]: |
|<font face="MBSarang Sattar" size="4">[[بنيادي چارج|برقي چارج]]: |
||
| سِٽَ 57: | سِٽَ 57: | ||
<font face="MBSarang Sattar" size="4">'''برقيو''' ھڪ بنيادي [[اڻوي ذرڙو]] آھي جيڪو ڪاٽُو ھڪ [[برقي چارج]] رکي ٿو. |
<font face="MBSarang Sattar" size="4">'''برقيو''' ھڪ بنيادي [[اڻوي ذرڙو]] آھي جيڪو ڪاٽُو ھڪ [[برقي چارج]] رکي ٿو. |
||
== سرسري نظر == |
|||
<font face="MBSarang Sattar" size="4">Within an [[اڻي]], the برقيا surround the [[اڻوي ناڀُ|ناڀ]] of [[پروٽيو]]۽ [[نِپُوسيو]]s in an [[electron configuration]]. The word ''electron'' was coined in [[1894]] and is derived from the term ''برقي زور'' introduced by [[وليم گلبرٽ]]. Its origin is the [[Greek language|يوناني]] word 'ηλεκτρον, meaning ''[[amber]]''. |
|||
Electrons in motion constitute [[برقي ڌار]], which may be used by scientists and engineers to measure many physical properties. Electric current existing for a finite time gives rise to a movement of charge ([[برقيت]]) that may be harnessed as a practical means to perform work. |
|||
The understanding of electrons has changed dramatically over the centuries, the most significant perhaps being the development of [[ذرڙاتي ميڪانيات]] in the 20th century and the idea of particle wave duality, that is, electrons can exhibit wave-like and particle like properties. Equally as important, [[particle physics]] has also furthered understanding of the electron immeasureably. |
|||
The variations in [[برقي اَڇَ]] generated by differing numbers of electrons and their configurations in atoms determine the chemical properties of the [[chemical element|elements]]. These fields play a fundamental role in [[ڪيميائي بانڊ]]s and [[ڪيميات]]. |
|||
== عملاً == |
|||
=== درجھ بندي === |
|||
The electron is one of a class of subatomic particles called [[ليپٽيا]], which are believed to be [[fundamental particle]]s (that is, they cannot be broken down into smaller constituent parts). The word "particle" is somewhat misleading, however, because [[ذرڙاتي ميڪانيات]] shows that electrons also behave like a wave, e.g., in the [[double-slit experiment]]; this is called [[wave-particle duality]]. |
|||
The antiparticle of an electron is the '''[[واڌويو]]''', which has the same mass but positive rather than negative charge. The term '''negatron''' is sometimes used to refer to standard electrons so that the term ''electron'' may be used to describe both positrons and negatrons, as proposed by [[Carl David Anderson|Carl D. Anderson]]. Under ordinary circumstances, however, ''electron'' refers to the negatively charged particle alone. |
|||
=== خصوصيتون ۽ روش === |
|||
Electrons have a negative [[برقي چارج]] of −1.6 × 10<small><sup>−19</sup></small> [[coulomb]]s, and a mass of about [[1 E-31 kg|9.11 × 10<small><sup>−31</sup></small> kg]] (0.51 MeV/c<sup>2</sup>), which is approximately <sup>1</sup>/<sub>1836</sub> of the mass of the [[proton]]. These are commonly represented as '''e<sup>−</sup>'''. |
|||
According to [[quantum mechanics]], electrons can be represented by [[لھري ڪارج]]s, from which the [[برقياتي گھاٽائي]] can be determined. Each electron has its own wavefunction, which is called an [[atomic orbital|orbital]]. The exact [[momentum]] and position of an electron cannot be simultaneously determined. This is a limitation described by the [[Heisenberg uncertainty principle]], which, in this instance, simply states that the more accurately we know a particle's position, the less accurately we can know its momentum, and vice versa. |
|||
The electron has [[spin (physics)|spin]] ½ and is a [[fermion]] (it obeys [[Fermi-Dirac statistics]]). In addition to its intrinsic angular momentum, an electron has a [[magnetic moment]] along its spin axis. |
|||
While most electrons are found in atoms, others move independently in matter, or together as an [[electron beam]] in a [[vacuum]]. In some [[superconductor]]s, electrons move in [[Cooper pair]]s, in which their motion is coupled to nearby matter via lattice vibrations called [[phonon]]s. When electrons move, free of the nuclei of atoms, and there is a [[net flow]], this flow is called [[electricity]], or an [[electric current]]. |
|||
A body has a static charge when the body has more or fewer electrons than are required to balance the positive charge of the nuclei. When there is an excess of electrons, the object is said to be negatively-charged. When there are fewer electrons than [[proton]]s, the object is said to be positively-charged. When the number of electrons and the number of protons are equal, their charges cancel each other and the object is said to be electrically neutral. A [[macroscopic]] body can acquire charge through rubbing, i.e., the [[phenomena]] of [[triboelectricity]]. Electrons and [[positron]]s can [[electron-positron annihilation|annihilate]] each other and produce a pair of [[photon]]s. However, high-energy photons may transform into an electron and a positron by a process called [[pair production]]. |
|||
The electron is an [[elementary particle]] — it has no [[substructure]] (at least, experiments have not found any so far, and there is good reason to believe that there is not any). Hence, it is usually described as [[point]]-like, i.e., with no [[spatial]] extension. However, if one gets very near an electron, one notices that its properties ([[charge]] and [[mass]]) seem to change. This is an effect common to all elementary particles: The particle influences the [[vacuum fluctuation]]s in its vicinity, so that the properties one observes from far away are the sum of the bare properties and the vacuum effects (see [[renormalization]]). |
|||
There is a physical constant called the [[classical electron radius]], with a value of 2.8179 × 10<sup>−15</sup> [[Metre|m]]. Note that this is the radius that one could infer from its charge if the physics were only described by the [[classical electromagnetism|classical]] theory of [[electrodynamics]] and there were no [[quantum mechanics]] (hence, it is an outdated concept that nevertheless sometimes still proves useful in calculations). |
|||
The speed of an electron in a [[vacuum]] can approach, but never reach c, the [[speed of light]] in a [[vacuum]]. This is due to an effect of [[special relativity]]. The effects of [[special relativity]] are based on a quantity known as [[gamma]] or the [[Lorentz factor]]. Gamma is a function of v, the velocity of the particle, and c. The following is the formula for gamma: |
|||
:<math>\gamma = 1 / \sqrt{1 - (v^2/c^2)}</math> |
|||
The energy necessary to accelerate a particle is [[gamma]] minus one times the rest mass. For example, the [[linear accelerator]] at [[Stanford]] can [http://www2.slac.stanford.edu/vvc/theory/relativity.html accelerate] an electron to roughly 51 GeV. This gives you a gamma of 100,000 given that the rest mass of an electron is 0.51 MeV/c² (the [[relativistic mass]] of this fast electron is 100 000 times its rest mass). Solving the equation above for the speed of the electron gives a speed of: |
|||
:<math>\left(1-\frac {1} {2} \gamma ^{-2}\right)c</math> = 0.999 999 999 95 c. |
|||
(The formula applies for large γ.) |
|||
=== ڪائنات ۾ === |
|||
It is believed that the number of electrons existing in the known [[universe]] is at least 10<sup>79</sup>. This number amounts to a density of about one electron per [[cubic metre]] of space. |
|||
Based on the [[classical electron radius]] and assuming a dense [[sphere packing]], it can be calculated that the number of electrons that would fit in the [[observable universe]] is on the order of 10<sup>130</sup>. Of course, this number is even less meaningful than the classical electron radius itself. |
|||
=== صنعت ۾ === |
|||
[[Electron beam]]s are used in [[electron beam welding|welding]] as well as [[electron beam lithography|lithography]]. |
|||
== تجربيگاھھ ۾ == |
|||
=== آڳاٽا تجربا === |
|||
The quantum or discrete nature of electron's charge was observed by [[Robert Millikan]] in the [[Oil-drop experiment]] of [[1909]]. |
|||
=== استعمال === |
|||
[[Electron microscope]]s are used to magnify details up to 500,000 times. Quantum effects of electrons are used in [[Scanning tunneling microscope]] to study features at the atomic scale. |
|||
== نظريي ۾ == |
|||
In relativistic [[quantum mechanics]], the electron is described by the [[Dirac Equation]]. [[Quantum electrodynamics]] (QED) models an electron as a charged particle surrounded by a sea of interacting [[virtual particles]], modifying the sea of [[virtual particles]] which makes up a vacuum. Although this theory involves difficult theoretical problems where calculations produce infinite terms, a practical (although mathematically dubious) method called [[renormalization]] was discovered whereby infinite terms can be cancelled to produce finite predictions about the electron. The correction of just over 0.1% to the predicted value of the electron's [[gyromagnetic ratio]] from exactly 2 (as predicted by Dirac's single particle model), and its extraordinarily precise agreement with the experimentally determined value, is viewed as one of the pinnacles of modern physics. There are now indications that [[string theory]] and its descendants may provide a model of the electron and other fundamental particles where the infinities in calculations do not appear, because the electron is no longer seen as a dimensionless point. At present, string theory is very much a 'work in progress' and lacks predictions analogous to those made by QED that can be experimentally verified. |
|||
In the [[Standard Model]] of [[particle physics]], it forms a doublet in SU(2) with the [[electron neutrino]], as they interact through the [[weak interaction]]. The electron has two more massive partners, with the same charge but different masses: the [[muon]] and the [[tau lepton]]. |
|||
The [[antimatter]] counterpart of the electron is its antiparticle, the [[positron]]. The positron has the same amount of electrical charge as the electron, except that the charge is positive. It has the same mass and spin as the electron. When an electron and a positron meet, they may [[Annihilation|annihilate]] each other, giving rise to two [[Gamma ray|gamma-ray]] photons, each having an energy of 0.511 [[MeV]] (511 [[keV]]). See also [[Electron-positron annihilation]]. |
|||
Electrons are also a key element in [[electromagnetism]], an approximate theory that is adequate for macroscopic systems, and for classical modelling of microscopic systems. |
|||
== تاريخ == |
|||
The electron as a unit of charge in electrochemistry was posited by [[G. Johnstone Stoney]] in [[1874]], who also coined usage of "electron" in [[1894]]. |
|||
The discovery that the electron was a [[subatomic particle]] was made in [[1897]] by [[J.J. Thomson]] at the [[Cavendish Laboratory]] at [[University of Cambridge|Cambridge University]], while he was studying "[[cathode rays]]". Influenced by the work of [[James Clerk Maxwell]], and the discovery of the [[X-ray]], he deduced that [[cathode ray tube|cathode rays]] existed and were negatively charged "''particles''", which he called "''corpuscles''." He published his discovery in [[1897]]. |
|||
The [[periodic law]] states that the chemical properties of elements largely repeat themselves periodically and is the foundation of the [[periodic table]] of elements. The law itself was initially explained by the [[atomic mass]] of the elements. However, as there were anomalies in the periodic table, efforts were made to find a better explanation for it. In [[1913]], [[Henry Moseley]] introduced the concept of the [[atomic number]] and explained the [[periodic law]] with the number of protons each element has. In the same year, [[Niels Bohr]] showed that electrons are the actual foundation of the table. In [[1916]], [[Gilbert Newton Lewis]] explained the chemical bonding of elements by electronic interactions. |
|||
== پڻ ڏسندا == |
|||
* [[معياري ماڊل]] |
|||
* [[اڻوي ذرڙا]] |
|||
* [[پروٽيو]] |
|||
* [[واڌويو]] |
|||
* [[ناڀيو]] |
|||
* [[Photoelectric Effect]] |
|||
* [[کنوڻ]] |
|||
* [[ذرڙن جي لسٽ]] |
|||
* [[Cathode rays]] |
|||
* [[برقيت]] |
|||
* [[فرميوي اَڇَ]] |
|||
== خارجي ڳندڍڻا == |
|||
* [http://www.aip.org/history/electron/ The Discovery of the Electron] from the American Institute of Physics History Center |
|||
* [http://pdg.lbl.gov/ Particle Data Group] |
|||
* Stoney, G. Johnstone, "''[http://dbhs.wvusd.k12.ca.us/webdocs/Chem-History/Stoney-1894.html Of the 'Electron,' or Atom of Electricity]''". Philosophical Magazine. Series 5, Volume 38, p. 418-420 October 1894. |
|||
* Eric Weisstein's World of Physics: [http://scienceworld.wolfram.com/physics/Electron.html Electron] |
|||
== حوالا == |
|||
* {{cite book | author=Griffiths, David J.|title=Introduction to Quantum Mechanics (2nd ed.) | publisher=Prentice Hall |year=2004 |id=ISBN 0-13-805326-X}} |
|||
* {{cite book | author=Tipler, Paul; Llewellyn, Ralph | title=Modern Physics (4th ed.) | publisher=W. H. Freeman | year=2002 | id=ISBN 0-7167-4345-0}} |
|||
* Brumfiel, G. ([[6 January]] [[2005]]). Can electrons do the splits? In ''Nature, 433'', 11. |
|||
</font> |
|||
{{Elementary}} |
|||
</font> |
|||
[[زمرو:Electron| ]] |
|||
[[زمرو:سائنس]] |
[[زمرو:سائنس]] |
||
ورجاءُ بمطابق 22:57, 22 اپريل 2012ع
| برقيو | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| درجھ بندي | ||||||||||||||
| ||||||||||||||
| خاصيتُون | ||||||||||||||
|
برقيو ھڪ بنيادي اڻوي ذرڙو آھي جيڪو ڪاٽُو ھڪ برقي چارج رکي ٿو.
